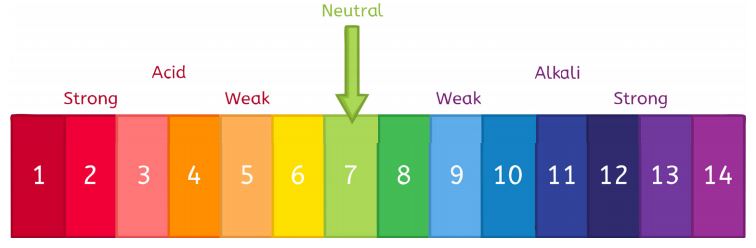
Today in science we are doing an experiment about neutralization. A Universal indicator - shows if acid or base. the colour shows us whether its an acid or a base. Blue, green, and red. (PH Scale) the acid we are using is hydrochloric acid and the base we are using today is called sodium hydroxide. An acid has a ph scale of 0-6 is the opposite of a base, which are sour. And a base is has a PH scale of 7-14. Lastly is a neutral area on the PH scale which is green in between the acidic side a the base side.
A chemical reaction occurs when you mix and acid and base together. the base cancels out the effect of the acid. this reaction is called a neutralisation reaction because a neutral solution is made up if you add around the right amount of acid and base together.
Equipment: a test tube. test tube rack, sodium hydroxide and hydrochloric acid, dropper or a dropper bottle, universal indicator solution.
Instructions:
1 add 2ml (2cm ish) of NACH into a test tube.
2 2 drops of universal indicator.
3 add drops of HCI until you reach a green colour.

Hey Tyra, the start of your blog with the summary of acids, bases, indicators and neutralisation is really great! Would be good to see an explanation of what you did? what changes happened? and what that shows you/why they happened?
ReplyDelete